Galvanic Reaction: Incompatible Metals Responsible for Corrosion
During a maintenance check in the 1980s, workers discovered that the Statue of Liberty had a serious structural issue: the statue’s internal wrought iron support system was rusting over because the insulating layer of shellac between the iron and copper had failed and allowed for galvanic corrosion to occur. Although this was not a surefire recipe for structural disaster, it was still a serious structural issue that took millions of dollars and months of work to ensure Lady Liberty’s structural integrity was better prepared to stand the test of time.
While atmospheric and crevice corrosion are some of the most common causes of metal deterioration in structural components, galvanic corrosion, another common culprit of metal deterioration, is just as widespread. Nonetheless, this pernicious chemical reaction is often misunderstood by many members of the construction industry.
As a designer, builder, engineer, or architect who works with exterior metals, you may have heard of the term galvanic corrosion (also known as bimetallic corrosion), or perhaps at the very least, are familiar with the concept of galvanized metals – such as steel coated with a thin layer of zinc to prevent rust – which employ a “controlled†form of galvanic corrosion to achieve their corrosion resistant properties.Â
While most people know that galvanic corrosion is dangerous, not many know how it works, how its real world consequences play out in terms of structural safety, and what can be done to avoid it.
This article provides important information about galvanic corrosion and related safety considerations.
Â
What is galvanic corrosion?
Simply speaking, galvanic corrosion is the damage or deterioration of metal that takes place between dissimilar metals because of an electrochemical reaction. Specifically, it occurs when two different metals come into contact with each other and have either been submerged or moistened by an electrolyte, with the corrosion taking place around the point where the two metals meet. Additionally, this reaction can be catalyzed by substances that increase the conductivity of water, like salt, and thus the rate of corrosion can vary based on the environment where the reaction takes place.
Galvanic corrosion occurs because each metal has its own electrical conductivity potential. This difference in electrode potential in turn drives a corrosive attack on the positively charged metal (anode), forcing it to dissolve into the electrolyte.
Most commonly, galvanic corrosion can be seen in plumbing systems where a copper pipe is directly connected to a steel or iron pipe. Once in contact both metals can undergo galvanic corrosion because of the electric or galvanic current that takes place at the anode and cathode of the pair of metals.
In addition to environmental salinity, the severity of galvanic corrosion that occurs when two metals come into contact is dependent on several other factors as well, including:
- The dissimilarity of the two participating metals and the difference between the electrode potentials of each of them.
- The surface of each of the metal and whether or not it has a protective film.
- The properties of the electrolyte, including the flow rate, volume, temperature, ionic species, conductivity, and pH.
- Presence of nearby concrete sealed with sodium acetate.
- The humidity, moisture, sun exposure, temperature variation, etc. of the local environment.
- Geometric and physical factors such as surface area, contact point, and the distance between the metals.
- Metallurgical properties such as the alloy mix, mechanical disturbance, and heat treatment.
- Other factors such as reversible electrode potentials, chemical reactions, and microbiological contributors.
Â
Examples of galvanic corrosion
In addition to the example involving the Statue of Liberty cited at the beginning of this article, there have been several other high-profile examples of galvanic corrosion affecting a structure’s integrity.
Another one of these famous examples involves St. Mary’s Cathedral in Tokyo, a prominent Catholic church built in 1964 with a unique metal design. In 2002, a photograph of the building was released, which showed its stainless steel roof, and while it was dirty, it was totally corrosion-free. However, just a few years later, the building’s roof peeled off during a storm. How did this happen? Well, in the intervening years the non-metal separator between the metal roof and metal structure deteriorated significantly. Once this inert separator was lost, galvanic corrosion caused the carbon steel support system to fail. Unfortunately, this is a common theme among lots of these prominent examples: designers often fail to realize that if a cladding or roof system is designed to last throughout the building’s life, then the same should apply to the structural support too.
Now of course not all of us do work involving ultra modern metal churches in Japan, so what are some more real-world examples of the safety implications of galvanic corrosion that the average architect or designer might face? Let’s say, for example, you want to build a stainless steel facade that’s fastened with screws. Now, if you opt for screws coated with zinc, then the stainless steel will aggressively corrode the zinc because fasteners coated with zinc are attacked by this dissimilar metal from all directions. Obviously this is not good: screws and anchors are vital to structural integrity and with the screws corroding, it could lead to structural failure. Not to mention the ugly rust trails and white corrosion that will bleed down the skin of the building as the metals corrode.
Therefore, it’s only a good idea to choose fasteners coated with zinc on metal if your metal is also the same or has similar nobility to that of zinc. In other words, you can choose zinc-coated fasteners for use with aluminum since it’s much closer to zinc on the galvanic series (a ranking of the molecular similarity of different metals), and therefore less dissimilar which in turn means there won’t be a lot of corrosion. However, you can completely avoid galvanic corrosion by choosing matching metal anchors. So, for example, choosing zinc on zinc would have the lowest risk for corrosion. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion:
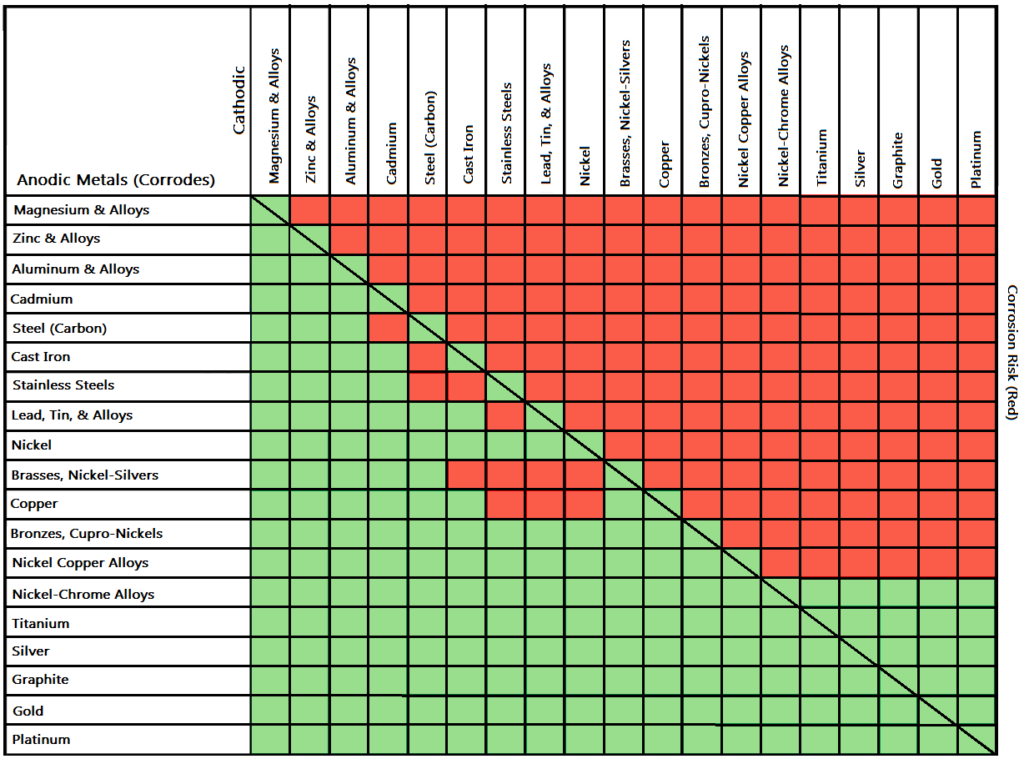
Â
How to prevent galvanic corrosion
While keeping the reactivity and nobility of metal structural materials in mind when choosing building components is one way to prevent corrosion from galvanic interaction, it isn’t the only way. It is possible to prevent galvanic reaction in metal structures and materials through the choice of proper building materials and components too. Here are some examples:
Â
- Preventing electrical connections: In cases where two dissimilar metals must be joined together, it’s best to separate them with any non-conductive component, like dielectric fittings on pipes.
- Making use of corrosion-resistant connectors: When joining two dissimilar metals, such as in cases where copper is used with iron pipes, it’s better to use a soldered or brazed joint instead of a threaded or mechanical one since the former is more durable than the latter.
- Choosing the right size or area of the joined metals: When it comes to joining two dissimilar metals, the higher-noble metal should have a smaller area, and the less-noble metal should have a larger area.
- Use antioxidants: when working with copper or aluminum use antioxidant pastes.
- Prevent electrolyte contact: coating metals with hydrophobic substances like grease prevents electrolyte contact, slowing any potential corrosion.
- Using protective coatings in the right manner: If you’re using an anti-corrosive paint or coating, then make sure that both of the metals are coated instead of just one.
- Using a sacrificial anode: In some cases, you can also cover the component with a material that serves as a “sacrificial anode.†So when corrosion does occur, it will only chip away the sacrificial anode and not harm the component underneath until all of the sacrificial anode has been corroded (as is the case with galvanized iron or steel which uses a thin layer of zinc as a sacrificial anode to prevent rust).
Â
Is galvanic corrosion always harmful?
While galvanic corrosion is generally something that is avoided at all costs, in some industries, a controlled galvanic reaction can be used for extending the asset’s life. Combining a metal to such as one that is higher on the galvanic series can spur the corrosion of the anode while protecting the anode’s cathodes that would have been at risk otherwise. This technique is known as cathodic protection, and while it is expensive, it’s a popular choice for hard-to-reach areas like buried pipelines or ships’ hulls.
Â
Conclusion
Galvanic corrosion is an expensive issue which can not only result in ugly rust stains and metal finishes, but is also a serious safety consideration that can lead to catastrophic and deadly structural failure if left unchecked. However, by taking a few measures, it’s possible to ensure that your buildings stay safe from galvanic corrosion. Remember to keep these factors in mind to help prevent and control galvanic reaction and ensure the safety and longevity of your building.
If you need further information about the corrosive properties of the metals we use in our products, please give us a call at (631) 750-3000.

